Abstract: Isotope-labeled relative and absolute quantification (iTRAQ) technology is a newly developed new proteomics quantitative research technology in recent years. The samples were also analyzed quantitatively. Researchers use iTRAQ protein quantification technology to accelerate protein quantification research. Of course, this emerging tool still needs further improvement.
Just knowing the identity of the protein is not enough to make a final conclusion about the protein, because the concentration of the protein is extremely important for achieving its function in the cell. A change in the concentration of a particular protein can predict the mutation process of the cell. Therefore, it is very important for scientists to be able to measure the relative and absolute concentration of proteins. In the past, scientists usually performed two-dimensional (2D) gel electrophoresis to cut off the band, and then used mass spectrometry to measure the protein in the band. However, this method is not ideal: it is neither very sensitive nor very precise.
Hong Li, director of the Proteomics Research Center at the New Jersey University of Medicine and Dentistry, said: "When we started proteomics research, we used 2D gel technology, but the amount of information obtained disappointed everyone because many proteins have changed. It has its own metabolic processes, such as heat shock proteins or housekeeping proteins. "
The methods in proteomics have been continuously improved. The tandem mass spectrometry method based on high sensitivity and accuracy does not require gels to obtain relative and absolute quantitative protein results. iTRAQ and iCAT are the two main forces in these new developments, but the new technology also has some unsatisfactory areas and needs to be continuously improved.
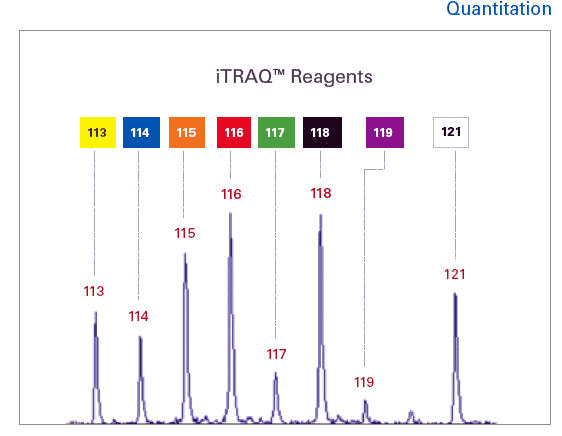
The iTRAQ kit includes four amine active reagents in the same amount, which can label protein-hydrolyzed peptides. Therefore, tandem mass spectrometry can be used to accurately identify and quantify peptides. Dominic Gostick, director of TOF-MS products, Proteomics, American Applied Biosystems, said: "The kit contains everything needed for the five-step reaction. It even includes insulin."
The operating procedures of iTRAQ are generally as follows. The protein is cleaved into peptides and then differentially labeled with iTRAQ reagent. The labeled samples are then mixed so that they can be compared. After combining with the sample, MudPIT multi-dimensional protein identification technology is usually used for the next operation, and 2D liquid chromatography tandem mass spectrometry is used for analysis. On the basis of mass spectrometry analysis to identify the structure of special peptide ion fragments, each peptide was identified using the software packages MASCOT and Protein Pilot of American Applied Biosystems.
Dr. Darryl Pappin, the inventor of iTRAQ, said that after hydrolysis, each protein will produce a large number of peptides. Each peptide is labeled with four iTRAQ reagents, each of which is regarded as an independent measurement, and all tests are uniform. However, some proteins may only be tested once, and it is up to the researchers to decide which peptides to continue.
iTRAQ identification
iTRAQ quantitative
Useful tool
Li believes that the iTRAQ test process is very simple, because the chemical labeling work is perfect. For quantitative work, the effect of labeling is important, and iTRAQ's quantitative work is repeatable. Because if a quantitative method cannot be repeated, it is meaningless.
Dr. Phillip Wright of the University of Sheffield in the United Kingdom believes that the reliability and repeatability of the technology are very good. The accuracy of iTRAQ is better than other tools, and the coefficient of variation obtained is in the range of 9%.
In order to investigate the coefficient of variation, the researchers verified the iTRAQ results using other methods. Dr. Jan Hirsch of the University of California in two different experiments verified the iTRAQ with an enzyme-linked immunosorbent assay (ELISA). He pointed out that the ELISA verification method has its own defects, it may not be able to detect all peptides detected by mass spectrometry, or the mass spectrometer may allocate additional peptides to proteins.
iTRAQ has more advantages than the previous technology. Hirsch believes that iTRAQ is a very good method in quantitative proteomics research, but he believes that further improvement is needed. The disadvantage of iCAT and iTRAQ technologies is that they only provide relative quantification. Therefore, only the relative abundance can be compared. In other words, the researchers can compare two sets of different samples, but for an individual protein concentration, they just get a relative value. Standardized controls can help quantify results, but often only provide information for one or a pair of proteins. In summary, the isotope mass spectrometry method can provide valuable information, such as the comparison of animal cell lysate samples after different treatments.
Dr. Beerelli Seshi of Harbor-UCLA Medical Center said: "Because we are interested in quantitative comparison, iTRAQ is more suitable for us because it can compare four samples at the same time. Not only can it be quantified, but it can also be identified."
iTRAQ is more sensitive than other protein quantification methods. Li said: "With iTRAQ technology, we have observed a large number of protein changes that were previously unseen." This is one of the problems with 2D gel electrophoresis because of its resolution and low sensitivity. Observing changes in low-abundance proteins requires highly sensitive properties.
Of course, as a global proteomics method, it should be objective. Seshi said that he has no preference for the research results. He uses iTRAQ to mark the proteome of bone marrow stromal cells and normal bone marrow stromal cells of leukemia patients growing in vitro. He hopes to study the content of the influence of the bone marrow microenvironment on the development of leukemia. Moreover, because cancer is a multi-gene disease, it is important to be able to study multiple proteins at once. Therefore, powerful proteomics methods are needed.
Another major advantage of iTRAQ is that it can identify any type of protein, including high molecular weight proteins, acidic proteins and basic proteins. 2D gel electrophoresis can't help these proteins. Moreover, 2D gel electrophoresis cannot analyze insoluble proteins like membrane proteins, but the combination of iTRAQ labeling system and mass spectrometry can solve these problems.
keep improving
Proteomics research has generated a lot of data. Seshi believes that the data is too large, so it needs to solve the data mining problem and use reasonable methods to analyze the data. He said: “The data output information does not include the basic information of protein, such as molecular weight and isoelectric point. The information obtained by tandem mass spectrometry analysis is much richer. "
Seshi believes that these richness make the data more complex, especially when studying the whole proteome. In his view, one of the disadvantages of proteomics technology is that they are not suitable for all proteomics, because high-abundance proteins interfere with the detection and identification of low-abundance proteins.
Wright said that mixing large amounts of proteomics data is another issue related to shotgun-type processing (such as iTRAQ): For each trial, researchers must identify the entire proteome. In the 2D gel electrophoresis method, one run of gel electrophoresis is followed by one imaging analysis, and then only one protein spot is selected for mass spectrometry analysis. 2D gel electrophoresis can not draw the entire proteome, but the difference can be observed.
iTRAQ is not only used for labeling the whole proteome, but also for the labeling of subcellular proteomes (such as organelles and membrane internal proteins). Dr. Alexandra Jones, the chief scientist of the Sainsbury laboratory in the United Kingdom, used iTRAQ in combination with phosphoconcentrated peptides to try to identify the phosphoproteome of Arabidopsis and the proteome information of plasma membranes. She first labeled it with iTRAQ. She said that the concentration process is very complicated and involves many steps, but once the proteome is labeled early, no matter how many operations are performed later, it becomes very simple.
Although there are some shortcomings, iTRAQ is still a powerful tool for proteomics research. Continue to improve its usable performance and standardize the technology, iTRAQ will definitely become a powerful weapon in proteomics research.
(Translated from "Genomics & Proteomics")
Cheer stick,banner,album,LOMO card,Brooch,Key chain,Doll
Shanghai Liuyuan Trading Co. , Ltd. , https://www.ly-weighing.com